STRANGE BUT TRUE- Odd reading: Odometer numbers raise questions
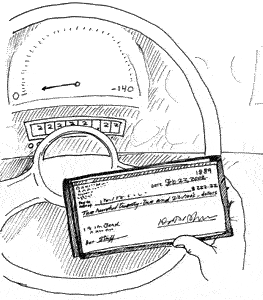
Q. My husband and I were driving when he happened to spot the odometer reading 22,222. Then we drove immediately to a store where we made multiple purchases and, yup, at the checkout the total was $222.22. We are wondering whether there's any way to figure the odds of the numbers coming up like this. –K. Rogers
A. There are lots of possible assumptions and answers, suggests University of California-Los Angeles mathematician James Ralston. For example, ignoring the decimal and assuming all five-digit numbers between 10000 ($100) and 99999 ($999.99) are equally likely, the chance is 1 in 90,000, since there are 90,000 such numbers.
If you broaden this to include the unlikelihoood of spotting the 22,222 on the odometer, things get even more interesting. Let's call these "monodigital" readings, says College of Charleston mathematician Alex Kasman. The probability of spotting one of these at any given time is small. Starting out, a car runs through 11 miles, 22, 33... 111, 222, 333... and so on. Drive 100,000 miles and you'll turn over 36 monodigitals. "They're certain to occur, though you probably won't notice most of them," Kasman says.
Now suppose you make a game of watching for these, and every time you spot one you pull over to make some random purchases. Can you reproduce the checkout feat of the Utah reader? Not likely, but not impossible either, says Kasman. If you spend $10 to $1000, there are four monodigitals– $22, $22.22, $222 and $222.22 out of 99,001 possible prices– to match 22,222 miles. Thus the probability is 4/99,001, or just a bit more than a one-in-25,000 chance. Probably the game will drive you crazy first...
Q. What's the current thinking on the old question of whether two snowflakes are ever exactly alike? –Frosty
A. All ice crystals are basically hexagonal, whether shaped as simple plates, bullets, needles, solid or hollow columns, dendrites, sheaths, says asktheweatherman.com.
Falling, they're a work-in-progress, riding air currents up and down, maybe for an hour or more, through regions of differing temperatures and humidities that leave their marks of growth and shape.
"Indeed, it is extremely unlikely that two complex snow crystals will look exactly alike," says California Institute of Technology physicist Kenneth G. Libbrecht. But how about two small crystals?
Consider that not all water molecules are alike, with about 1/1000 being atypical. Since even a small snow crystal has about a thousand million billion (18 zeros) water molecules, about a million billion (15 zeros) will be these "rogues," scattered throughout in unique ways.
So given a trillion trillion (24 zeros) crystals/year falling on Earth, the chance of two ever having the same water molecule layout is essentially zero!
The only exception would be tiny crystals with only maybe 10 molecules, all of which just might fit identically to some other crystal. "But they'd have to be assembled in a lab to be seen, and would be invisible to the naked eye."
Q. Why is ice so slippery? Better watch your step on this one... a) pressure melting puts water underfoot; b) frictional heating under skis or skates creates the glide; c) it's just ice's icy nature. –H. Brinker
A. a) and b) used to be given, but it's now known that the pressure under a walker's boots or the friction of skate blades against ice doesn't play much of a role in melting, reports Physics Today.
As early as the 1850s Michael Faraday tested ice cubes that were sticking together and concluded that ice surfaces must just inherently consist of a thin film of water, at whatever temperature. So mark c).
Even ice at -200 F has a "quasi-fluid layer" that makes the stuff slippery, says exploratorium.edu. This may help explain the "fast ice" and "slow ice" of hockey: As the ice gets warmed, the number of slippery layers increases, until skaters need to "slosh" through so many layers that the friction slows them down. On the other hand, these extra layers can help "soften" the landing of a figure skater, whose ice should be warmer than for hockey players.
By the time ice gets down to -250 F (-157 C), the slippery layer is just a single molecule thick! Nanohockey, anyone?
Q. Who sent the world's first email? -A. Gore
A. If the question means "who sent the first message over a network from one computer to another computer?" then Len Kleinrock– UCLA professor and one of the inventors of packet switching– lays claim to this, though it is not known for sure, says computer scientist Alan Kay of UCLA, MIT and Kyoto University. This was done on the old ARPAnet, which became the Internet and which first started up in September 1969.
However, sending messages to others, including instant messaging and chat, appeared much earlier on the first time-sharing systems, says Kay. These were big mainframe computers that could handle 100 or more users at a time communicating with the mainframe via typewriter terminals. One of the earliest was CTSS at MIT, circa 1963. Another was Project Genie at Berkeley. Both had email, IM, and chat. So why did it take so long for email to catch on?
"Quite a few people have to believe something is normal before it becomes normal– a sort of 'voting' situation. But once the threshold is reached, then everyone demands to do whatever it is," Kay says.
Gotta go now to check our email.
Send Strange questions to brothers Bill and Rich at , coauthors of "Can a Guy Get Pregnant? Scientific Answers to Everyday (and Not-So- Everyday) Questions," from Pi Press.
#